-
Templates
1099 FormsAccurately report 1099 information returns and ensure IRS filing with easeExplore all templatesW-9 W-8 FormsEasily manage and share taxpayer details to streamline payments and meet IRS requirements with confidenceExplore all templatesOther Tax FormsFillable tax forms simplify and speed up your tax filing process and aid with recordkeeping.Explore all templatesReal EstateReal estate templates for all cases, from sale to rentals, save you a lot of time and effort.Explore all templatesLogisticsSimplify your trucking and logistics paperwork with our ready-to-use transportation and freight templates.Explore all templatesMedicalMedical forms help you keep patient documentation organized and secure.Explore all templatesBill of SaleBill of Sale templates streamline the transfer of ownership with clarity and protection.Explore all templatesContractsVarious contract templates ensure efficient and clear legal transactions.Explore all templatesEducationEducational forms and templates enhance the learning experience and student management.Explore all templates
-
Features
FeaturesAI-Enhanced Document Solutions for Contractor-Client Success and IRS ComplianceExplore all featuresAI Summarizer Check out the featureAI PDF summarizer makes your document workflow even faster. Ask AI to summarize PDF, assist you with tax forms, complete assignments, and more using just one tool.Sign PDF Check out the featurePDFLiner gives the opportunity to sign documents online, save them, send at once by email or print. Register now, upload your document and e-sign it onlineFill Out PDF Check out the featurePDFLiner provides different tools for filling in PDF forms. All you need is to register, upload the necessary document and start filling it out.Draw on a PDF Check out the featureDraw lines, circles, and other drawings on PDF using tools of PDFLiner online. Streamline your document editing process, speeding up your productivity
- Solutions
- Features
- Blog
- Support
- Pricing
- Log in
- Sign Up
Medical Forms
-
 Doctor Note for Work
What Is Doctor Note for Work
Fillable doctors note also known as doctor’s excuse letter is the form that is provided by the doctor to the patient whenever it is required by the employer. It is an official document that explains a day or several days
Doctor Note for Work
What Is Doctor Note for Work
Fillable doctors note also known as doctor’s excuse letter is the form that is provided by the doctor to the patient whenever it is required by the employer. It is an official document that explains a day or several days
-
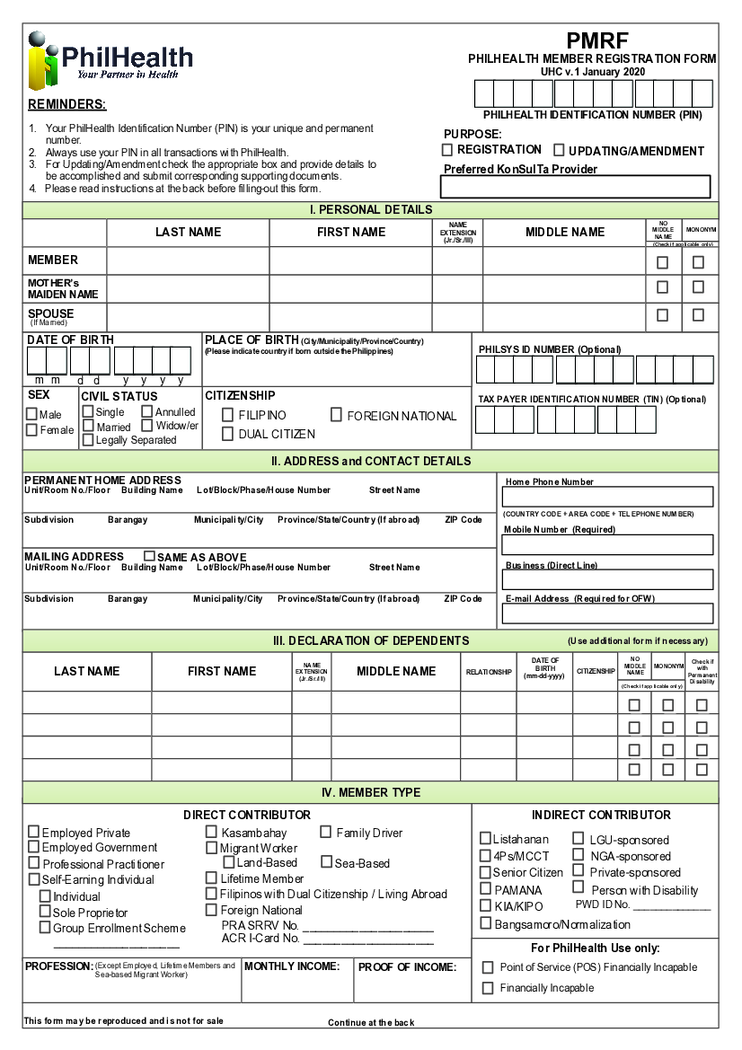 PMRF Form
What is a PMRF Form
The PMRF Form of PhilHealth is a two-page application that Filipino citizens can use to become a member of the local insurance program. You need to obtain a PhilHealth Identification Number before filling it out. You do not need other
PMRF Form
What is a PMRF Form
The PMRF Form of PhilHealth is a two-page application that Filipino citizens can use to become a member of the local insurance program. You need to obtain a PhilHealth Identification Number before filling it out. You do not need other
-
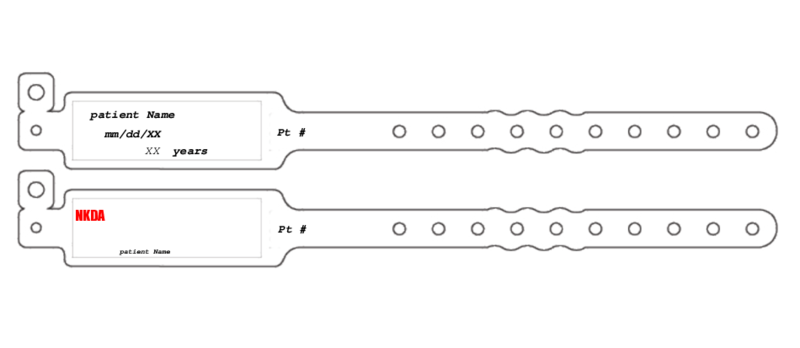 Patient Wristband Template
What Is a Patient Wristband Template?
A patient wristband template is a fillable blank form to be used when generating an informative wristband for inpatients. The primary purpose of this form is to make essential medical information visible.
W
Patient Wristband Template
What Is a Patient Wristband Template?
A patient wristband template is a fillable blank form to be used when generating an informative wristband for inpatients. The primary purpose of this form is to make essential medical information visible.
W
-
 Miscarriage Doctor Note
What Is a Miscarriage Doctor’s Note?
A doctor's note about a miscarriage is a document that says a woman has had a miscarriage and needs time to recover. Certificate usually states how long the woman needs to recover and may offer other recommen
Miscarriage Doctor Note
What Is a Miscarriage Doctor’s Note?
A doctor's note about a miscarriage is a document that says a woman has had a miscarriage and needs time to recover. Certificate usually states how long the woman needs to recover and may offer other recommen
-
 Work or School Medical Excuse
What Is Work and School Medical Excuse?
You need doctors work excuse either to show up at work or at school to explain the missed days. This document officially confirms that you need some time off your studies or work based on your current physical condi
Work or School Medical Excuse
What Is Work and School Medical Excuse?
You need doctors work excuse either to show up at work or at school to explain the missed days. This document officially confirms that you need some time off your studies or work based on your current physical condi
-
 COVID-19 Doctor Note
What Is COVID-19 Doctor Note Template?
Covid-19 doctor note template is also called a letter from a Health Care professional supporting workplace requests for accommodations related to COVID-19. This is the document that suggests you need to stay at home
COVID-19 Doctor Note
What Is COVID-19 Doctor Note Template?
Covid-19 doctor note template is also called a letter from a Health Care professional supporting workplace requests for accommodations related to COVID-19. This is the document that suggests you need to stay at home
-
 Doctor Return to Work Note
What Is a Return to Work Note From a Doctor?
Doctor Return to Work Note is a form that confirms or disclaims your ability to get back to work after some time that you were absent because of your health status. It also points to some physical restrictions
Doctor Return to Work Note
What Is a Return to Work Note From a Doctor?
Doctor Return to Work Note is a form that confirms or disclaims your ability to get back to work after some time that you were absent because of your health status. It also points to some physical restrictions
-
 Prisma Health Doctors Note Form
What Is a Prisma Health Doctors Note
The Prisma Health Doctor's Note is an official medical document issued by healthcare specialists affiliated with Prisma Health. Its primary purpose is to provide a formal and legitimate record of a patient's me
Prisma Health Doctors Note Form
What Is a Prisma Health Doctors Note
The Prisma Health Doctor's Note is an official medical document issued by healthcare specialists affiliated with Prisma Health. Its primary purpose is to provide a formal and legitimate record of a patient's me
-
 STD Testing Results Form
What Is an STD Testing Results Form?
The printable blank STD test result form is used to protect your health by diagnosing venereal diseases. Sexually transmitted diseases (STDs) can be conveyed through any sexual activity. This specific PDF for
STD Testing Results Form
What Is an STD Testing Results Form?
The printable blank STD test result form is used to protect your health by diagnosing venereal diseases. Sexually transmitted diseases (STDs) can be conveyed through any sexual activity. This specific PDF for
-
 Medicare enrolment form (MS004)
What Is Medicare Enrolment Form MS004
It’s a document designed to facilitate enrollment in the Medicare program, Australia's national healthcare system. Its purpose is to collect vital information from individuals seeking Medicare coverage, incl
Medicare enrolment form (MS004)
What Is Medicare Enrolment Form MS004
It’s a document designed to facilitate enrollment in the Medicare program, Australia's national healthcare system. Its purpose is to collect vital information from individuals seeking Medicare coverage, incl
-
 CDPH 530 Form
Understanding CDPH 530
The Controlled Document of Public Health, otherwise known as CDPH 530, is a crucial form focused on maintaining adequate nurse staffing assignment in healthcare facilities. It involves the meticulous allocation of work based on the
CDPH 530 Form
Understanding CDPH 530
The Controlled Document of Public Health, otherwise known as CDPH 530, is a crucial form focused on maintaining adequate nurse staffing assignment in healthcare facilities. It involves the meticulous allocation of work based on the
-
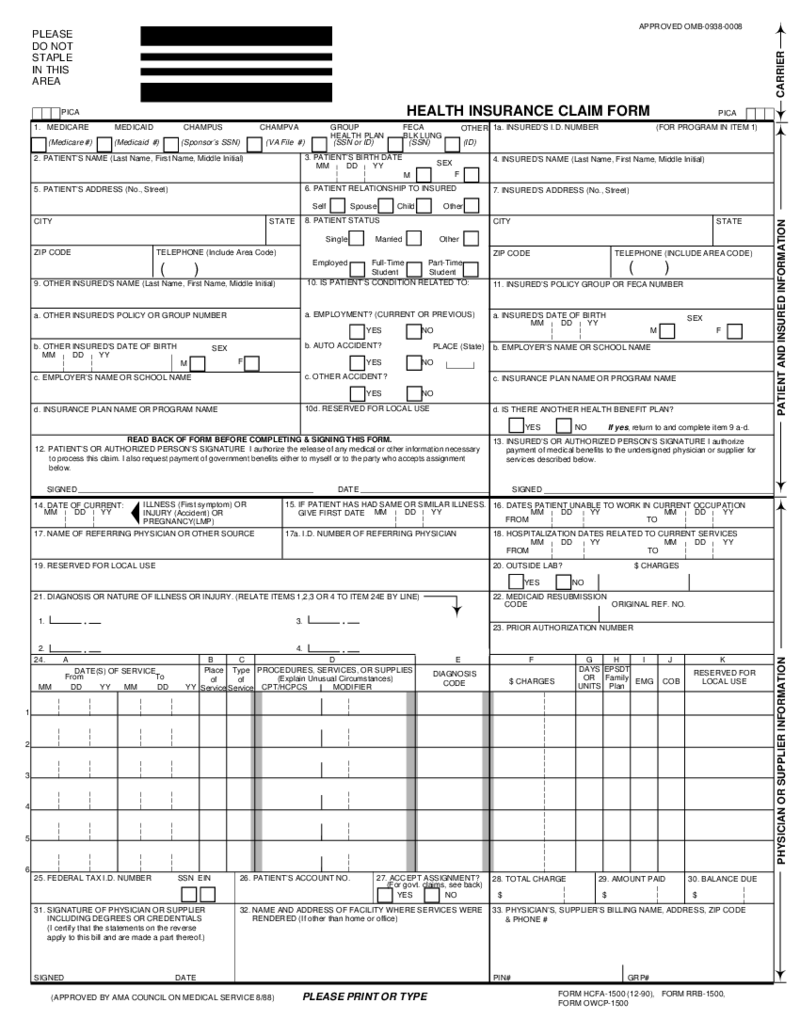 HCFA 1500 Form (12-90)
What Is an HCFA 1500 Form
The HCFA claim 1500 form (12-90), also referred to as CMS-1500, is a previous version of a standardized document used by healthcare providers to bill insurance com
HCFA 1500 Form (12-90)
What Is an HCFA 1500 Form
The HCFA claim 1500 form (12-90), also referred to as CMS-1500, is a previous version of a standardized document used by healthcare providers to bill insurance com
What Are Medical Forms Used For?
You can use medical forms for a variety of purposes. Generally you will use forms to create custom medical forms, track patient information, or maintain medical records. Medical templates may help you create printable medical charts and graphs.
What Are Medical Templates?
Medical form templates are pre-designed documents that help you save time. Using a medical template, you could simply fill out information and get a professional-looking copy in minutes.
When using a medical template, you should remember to customize form for your specific needs. If you creating a progress note, you should definitely include all the necessary patient information. When creating a referral letter, you should include the correct contact information for the referral.
Medical Form Templates Types
You can find numerous types of medical form templates on the Internet. Some templates are for specific medical procedures, while others are generic and can be used for any medical form.
- Medical Progress Template
Medical progress template is a type of medical template you may use to document a patient's progress during treatment. This form is one that doctors, nurses, and other healthcare providers can use to track a patient's progress and provide the best possible care.
- Medical History Form
A medical history form is a type of medical template that documents a patient's medical history. Usually, this form helps when a patient visits a new doctor for the first time or when a doctor prescribes a new medication.
- Medication List
A medication list is a type of medical template that lists all of the medications the patient is currently taking.
- Medical Referral Form Template
A medical referral form uses when requesting an appointment or consultation with a medical specialist or other health care provider.
- Medical Invoice Template.
A medical invoice template is a type of template you use to create invoices for medical services. These templates usually include fields for the date, service rendered, patient name, and amount owed. Some medical billing templates may also include a field for the provider's name or logo.
- Patient Sign-In Sheet Template
You can use this patient sign-up sheet template to collect contact information for new patients or update information for existing patients.
What Should Include in Medical Templates?
Medical templates are an important part of any medical office. They help keep track of patients, appointments, and medical records. Numerous different types of medical record forms, but there are some common elements that should be included in all medical templates:
- Patient Information: This is the most important part of any medical template. Patient information should include the patient's name, address, phone number, date of birth, and medical history.
- Appointment Information: Here you should include the date, time, and location of the appointment. It should also include the name and contact information of the doctor or other healthcare provider who will see the patient.
- Insurance Information: Next, you should include information about the patient's insurance, like the name of the insurance company, policy number, and contact information for the insurance company.
- Information about medications: Here should be a list of all the medications the patient is taking, along with the dosage and frequency of each medication.
- Allergies and Reactions: Allergies or reactions to medications, food, or other substances should be listed in this section.
- Immunizations: This part should include a list of any immunizations the patient has had.
- Test Results: Include a list of any test results the patient has received, such as blood tests, x-rays, and MRI results.
- Treatment Plans: A list of all treatment plans the patient is currently following, such as physical therapy, occupational therapy or surgery, should be included in this section.
- Billing Information: This section should include a list of all charges for the patient's treatment as well as insurance coverage and any copayments or deductibles due.
- Signature: Place for the patient's signature as well as the date should be in this section. This signature indicates that the patient has received a copy of the medical form and understands the information it contains.
How to Create Medical Templates: Step by Step
In order to create online medical templates, you need to follow these steps:
- Choose the type of template you want to create. There are many different types of medical templates, so it is important to choose the one that is right for you.
- Fill out the medical template you like. You may need to add or remove information from the template. You can find free printed medical forms at PDFliner or in medical books.
- Save the template. You need to save the template so you can use it in the future.
How to Fill Out Medical Forms?
Filling out medical forms may be a daunting task, but providing accurate and complete information is important in order to provide you with the best possible care. Here are some tips to help you fill out medical forms:
- Read the Instructions Carefully: First, make sure you understand what information is being requested and how it should be provided.
- Gather All the Information You Need: Further, before you start filling out the medical template, make sure you have all information you need, including personal and medical history, contact information, insurance information, and relevant documents.
- Use Legible Handwriting: If you are filling out a form by hand, please use clear, legible handwriting so your information is legible.
- Provide Complete and Accurate Information: Giving complete and accurate details on medical forms is important because it will help your healthcare provider make informed decisions about your treatment.
Remember, the more complete and accurate the information you provide on medical forms templates, the better your healthcare provider will be able to understand your medical history and needs and the better equipped they will be to provide you with the best possible care.
-
How long do you keep medical release forms?
The length of time that medical release forms should be kept can vary depending on the specific circumstances and the laws and regulations in your location. In general, it is a good idea to keep medical release forms for at least as long as the information on the form is relevant and may be needed for future medical treatment or care.


























































